bombbudpuffa
Sweet Cheeba Chiefa
- Joined
- Oct 28, 2006
- Messages
- 5,880
- Reaction score
- 4,347
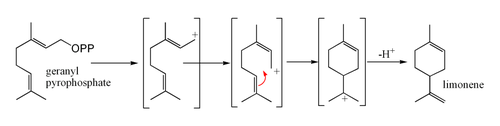
What is d-Limonene?
d-Limonene: A Cleaner from Nature
d-Limonene is the major component of the oil extracted from citrus rind. When citrus fruits are juiced, the oil is pressed out of the rind. This oil is separated from the juice, and distilled to recover certain flavor and fragrance compounds. The bulk of the oil is left behind and collected. This is food grade d-Limonene. After the juicing process, the peels are conveyed to a steam extractor. This extracts more of the oil from the peel. When the steam is condensed, a layer of oil floats on the surface of the condensed water. This is technical grade d-Limonene.
In the past decade, the use of d-Limonene has expanded tremendously. Much of the product goes into making paint solids, used to impart an orange fragrance to products, and used as a secondary cooling fluid. But the largest growth segment has been the use of d-Limonene in cleaning products. This has occurred in both industrial uses and in household/institutional products. d-Limonene can be used either as a straight solvent, or as a water dilutable product.
As a straight solvent, d-Limonene can replace a wide variety of products, including mineral spirits, methyl ethyl ketone, acetone, toluene, glycol ethers, and of course fluorinated and chlorinated organic solvents. As with most organic solvents, d-Limonene is not water soluble, so it can be used in the typical water separation units. With a KB value of 67, d-Limonene has solubility properties close to that of CFCs, indicating that it is a much better solvent than a typical mineral spirit. Straight d-Limonene can be used as a wipe cleaner, in a dip bath, or in spray systems as a direct substitute for most other organic solvents.
By combining d-Limonene with a surfactant package, a water diluting and rinsible solution can be made. In most cases these products are used in the institutional and household settings in place of caustic and other water based cleaners. A concentrated solution of a d-Limonene/surfactant solution can be made to be diluted before use, or pre-diluted solutions can be formed. The use concentrations of d-Limonene in these situations are usually 5-15%. In general these solutions are used as spray and wipe cleaners. The water dilutable solutions can also be used in industrial settings where a water rinse of the parts is desired to remove any residue which may remain.
d-Limonene is a very versatile chemical which can be used in a wide variety of applications. It is extremely safe and more effective than typical cleaning solutions.

How do we get d-Limonene and Orange Oil?
d-Limonene is the major component of the oil extracted from the citrus rind during the citrus juicing process. When the fruit is juiced, the oil is pressed out of the rind, then separated from the juice and distilled to recover certain flavor and fragrance compounds.
After the juicing process, the peels are conveyed to a steam extractor. When the steam is condensed, a layer of oil floats on the surface of the condensed water. This removes the bulk of the oil from the peel.
Food grade d-Limonene is extracted through the juicing process and Technical grade d-Limonene is removed through the peel steam extraction process. The chart below shows the citrus oil manufacturing process and the specific oils that come from each part of the process.
hxxp://www.floridachemical.com/whatisd-limonene.htm
My question is can this be used or applied in a way that would benefit taste and smell of mj(food grade, of course)?
d-Limonene: A Cleaner from Nature
d-Limonene is the major component of the oil extracted from citrus rind. When citrus fruits are juiced, the oil is pressed out of the rind. This oil is separated from the juice, and distilled to recover certain flavor and fragrance compounds. The bulk of the oil is left behind and collected. This is food grade d-Limonene. After the juicing process, the peels are conveyed to a steam extractor. This extracts more of the oil from the peel. When the steam is condensed, a layer of oil floats on the surface of the condensed water. This is technical grade d-Limonene.
In the past decade, the use of d-Limonene has expanded tremendously. Much of the product goes into making paint solids, used to impart an orange fragrance to products, and used as a secondary cooling fluid. But the largest growth segment has been the use of d-Limonene in cleaning products. This has occurred in both industrial uses and in household/institutional products. d-Limonene can be used either as a straight solvent, or as a water dilutable product.
As a straight solvent, d-Limonene can replace a wide variety of products, including mineral spirits, methyl ethyl ketone, acetone, toluene, glycol ethers, and of course fluorinated and chlorinated organic solvents. As with most organic solvents, d-Limonene is not water soluble, so it can be used in the typical water separation units. With a KB value of 67, d-Limonene has solubility properties close to that of CFCs, indicating that it is a much better solvent than a typical mineral spirit. Straight d-Limonene can be used as a wipe cleaner, in a dip bath, or in spray systems as a direct substitute for most other organic solvents.
By combining d-Limonene with a surfactant package, a water diluting and rinsible solution can be made. In most cases these products are used in the institutional and household settings in place of caustic and other water based cleaners. A concentrated solution of a d-Limonene/surfactant solution can be made to be diluted before use, or pre-diluted solutions can be formed. The use concentrations of d-Limonene in these situations are usually 5-15%. In general these solutions are used as spray and wipe cleaners. The water dilutable solutions can also be used in industrial settings where a water rinse of the parts is desired to remove any residue which may remain.
d-Limonene is a very versatile chemical which can be used in a wide variety of applications. It is extremely safe and more effective than typical cleaning solutions.

How do we get d-Limonene and Orange Oil?
d-Limonene is the major component of the oil extracted from the citrus rind during the citrus juicing process. When the fruit is juiced, the oil is pressed out of the rind, then separated from the juice and distilled to recover certain flavor and fragrance compounds.
After the juicing process, the peels are conveyed to a steam extractor. When the steam is condensed, a layer of oil floats on the surface of the condensed water. This removes the bulk of the oil from the peel.
Food grade d-Limonene is extracted through the juicing process and Technical grade d-Limonene is removed through the peel steam extraction process. The chart below shows the citrus oil manufacturing process and the specific oils that come from each part of the process.
hxxp://www.floridachemical.com/whatisd-limonene.htm
My question is can this be used or applied in a way that would benefit taste and smell of mj(food grade, of course)?